
The latest market publication titled Atopic Dermatitis Market Size, Share and Trends Analysis by Region, Drug Class, Route of Administration, and Segment Forecast, 2022-2027 has been added to the report store by GlobalData Plc. The AD market growth is likely driven by a recent wave of biological therapies prescribed for treating AD. Furthermore, the rising prevalence of atopic dermatitis and surging demand for biologics in atopic dermatitis will further proliferate the market’s growth during the forecast period.
Read our FREE Sample Report for more market dynamics
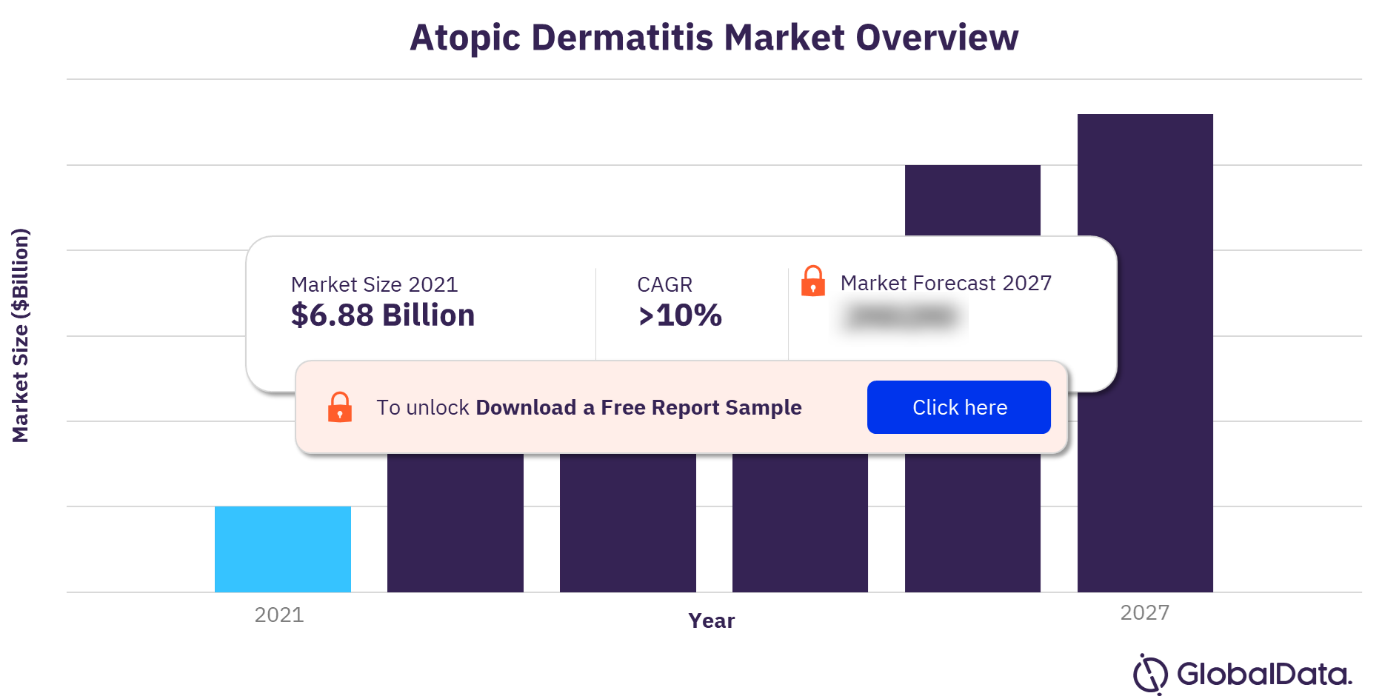
The atopic dermatitis market research report offers a thorough, forward-looking analysis of the atopic dermatitis market and key opportunities in a concise format to help executives build proactive and profitable growth strategies. The AD market growth will also witness positive growth during the projected period mainly due to the trend among major players about the constant competition to launch new drugs. However, the AD market analysis report predicts factors such as high treatment costs and lack of reimbursement guidelines in developing countries coupled with an increase in hypersensitive reactions leading to product recalls that limit market growth during the next few years.
Request our Sample PDF for information and insights on growth factors
Atopic Dermatitis Market Segment Highlights
By Drug Class
- Corticosteroids: The corticosteroids segment held the largest atopic dermatitis market share in 2021. The segment will continue to account for the highest growth throughout the forecast period owing to the broad prevalence and the first line of defence for the treatment of the ailment under discussion. Doctors advise using corticosteroid gels or ointments to start the treatment in accordance with the preliminary findings.
- Calcineurin Inhibitors
- PDE4 Inhibitors
- Biologics
- Others
By Route of Administration
- Injectable: Doctors advise using corticosteroid gels or ointments to start the treatment in accordance with the preliminary findings. Dupilumab development program is one of the clinical initiatives intended to develop AD medications for pediatric patients. The program’s objective is to enhance clinical research. Some injectables in the treatment of AD belong to the immunosuppressants category. Immunosuppressants reduce itching, inflammation, and issues with the skin barrier by inhibiting the immune system from triggering the inflammatory skin response that is characteristic of atopic dermatitis
- Oral
- Topical
Regional Opportunities
- North America: North America leads the atopic dermatitis market in 2021 owing to its fast-paced development and commercialization potential for advanced technologies, and growing developments in biopharmaceutical and research. In this region, the prevalence of AD in the adult group is growing much faster for several reasons, such as most therapies being initially developed for use in adults and then expanding into younger patients. In addition, physicians are usually more conservative with their treatment of younger patients due to uncertainty surrounding long-term safety and efficacy.
- Europe
- Asia-Pacific
- ROW
For more segment-wise insights and regional opportunities, Download Sample PDF
Atopic Dermatitis Market Vendor Landscape
The AD market competitive landscape is witnessing the emergence of competition with existing players and emerging start-ups. Companies are launching innovative solutions and products to garner significant market share throughout the forecast period.
Top Atopic Dermatitis Market Players
- Sanofi: The company is engaged in the discovery, development, manufacturing, and marketing of a wide range of medicines and vaccines. Sanofi’s R&D efforts focus on advancing a combination drug to increase the effectiveness of treatments and on advancing the formulation of new biologics to produce precision medicines. In September 2022, Sanofi announced positive results published in the Lancet from a Phase 3 Dupixent (dupilumab) trial in children aged 6 months to 5 years.
- Pfizer Inc.: The company discovers, develops, manufactures, and commercializes biopharmaceuticals. The company offers products to treat various conditions such as cardiovascular, metabolic and pain, cancer, inflammation, immune disorders, and rare diseases. The company’s key product includes CIBINQO. In December 2021, Pfizer Inc. announced the European Commission (EC) approval for its100 mg and 200 mg doses of Cibinqo, a Janus kinase 1 (JAK1) inhibitor, for the treating moderate-to-severe atopic dermatitis.
- AbbVie Inc.: AbbVie Inc (AbbVie) is a specialty biopharmaceutical company, which discovers, develops, manufactures, and commercializes drugs for the treatment of chronic and complex diseases. The company’s key products include Rinvoq, which is indicated in the treatment of moderate to severely active rheumatoid arthritis in adult patients who have poorly responded to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs).
Grab your Sample Report Copy for vendor-specific offerings and strategic initiatives
About GlobalData
GlobalData is a leading data, analytics, and insights provider in the world’s largest industries. As a leading information services company, thousands of clients rely on GlobalData for trusted, timely, and actionable intelligence. Our mission is to help our clientele ranging from professionals within corporations, financial institutions, professional services, and government agencies to decode the future and profit from faster, more informed decisions. Continuously enriching 50+ terabytes of unique data and leveraging the collective expertise of over 2,000 in-house industry analysts, data scientists, and journalists, as well as a global community of industry professionals, we aim to provide decision-makers with timely, actionable insights.
Media Contacts
Mark Jephcott
Head of PR EMEA
mark.jephcott@globaldata.com
cc: pr@globaldata.com
+44 (0)207 936 6400






