- GATHER2 Enrollment Nears Completion; Timeline Accelerated to Late July of this Year -
- GATHER2 Retention Exceeding Expectations; with Injection Fidelity Rate Target for GATHER2 Greater than 90% -
- GATHER1 18 Month Post-Hoc Analyses Show that Zimura 2 mg Has the Potential to Have an Impact on Earlier Stages of Dry AMD Prior to Geographic Atrophy -
- 19.6% Reduction in Rate of Progression from Drusen to iRORA/cRORA as Compared to Sham at 18 Months Representing a Relative Risk Reduction of 72% -
- 21.8% Reduction in Rate of Progression from iRORA to cRORA as Compared to Sham at 18 Months Representing a Relative Risk Reduction of 52% -
- Webcast Today, June 18, 2021 to Begin at 10:00 a.m. Eastern Time -
IVERIC bio, Inc. (Nasdaq: ISEE) announced that today at the Company’s Virtual Symposium for Investors and Analysts, Arshad M. Khanani, MD, MA, of Sierra Eye Associates and Chairman of the GATHER2 Steering Committee, will discuss an accelerated enrollment timeline and patient retention, including injection fidelity, for GATHER2, the Company’s pivotal clinical trial of Zimura® (avacincaptad pegol) in development for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The Company expects to complete enrollment in GATHER2 in late July of this year. Based on this timeline, the Company expects topline GATHER2 data to be available in the second half of 2022, approximately one year after the enrollment of the last patient plus the time needed for database lock and analysis. The Company also announced that GATHER2 is exceeding patient retention expectations. The Company is targeting patient retention for the trial, as measured by injection fidelity rate through month 12, of greater than 90%. Injection fidelity is calculated by dividing the total number of actual injections by the total number of expected injections based on the number of enrolled patients.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210618005081/en/
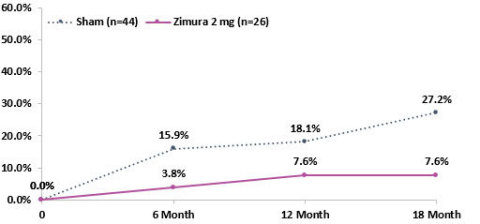
Proportion of Patients that progress from drusen to iRORA or cRORA (Zimura 2 mg vs. Sham)
“Since the initiation of GATHER2, Iveric Bio has implemented a patient centric strategy with multiple initiatives to tackle the many challenges that the COVID-19 pandemic brought to conducting clinical trials,” stated Dr. Khanani. “It is exciting to be a part of a clinical trial that is exceeding enrollment and retention targets and timelines in the midst of a global pandemic. I believe that the positive results from GATHER1, including the early and continuous treatment effect demonstrated, is a key motivator for the recruitment and retention in the GATHER2 clinical trial.”
“We are thrilled to have world leading retinal specialists participate in our symposium and to share the new post-hoc analyses of GATHER1 and the progress of GATHER2,” stated Dhaval Desai, PharmD, Chief Development Officer of Iveric Bio. “Thus far we have seen an injection fidelity rate well above our target goal of greater than 90% and ahead of our expectations. We consider injection fidelity to be the most important component of patient retention because it reflects the timely administration of the drug into the patient’s eye. We continue to focus as much on retention as recruitment, not only to protect the integrity of our data, but also to potentially demonstrate the early and continuous treatment effect observed in GATHER1.”
The Company also announced that at today’s event, Vas Sadda, MD, of Doheny Eye Institute at UCLA, will present new post-hoc analyses from the GATHER1 clinical trial on progression of drusen and nascent GA (iRORA*/cRORA**), which are earlier forms of dry AMD, in patients treated with Zimura 2 mg as compared to patients in the sham group. The accompanying graphs illustrate the data that Dr. Sadda will discuss today.
Dr. Sadda stated, “I am excited to present these encouraging data. Dry AMD is the most common cause of blindness in the US, but we have no approved treatments for this devastating disease. Some drugs, including Zimura, are being studied to decrease the rate of growth of geographic atrophy, which is very important. However, the significance of these post-hoc analyses suggest that Zimura may have the potential to impact the disease even before atrophy occurs. Given the compelling results, I believe prospective, randomized studies with Zimura on patients with earlier stages of dry AMD are warranted.”
Pravin Dugel, MD, President of Iveric Bio, stated, “In the GATHER1 post-hoc analyses, we reported decreased conversion of iRORA to cRORA and a decreased conversion of drusen to iRORA or cRORA. Both rates showed an increasing effect over time, consistent with Zimura’s effect on geographic atrophy in GATHER1. While the former shows that Zimura may have a therapeutic benefit in earlier stages of geographic atrophy, the latter suggests that Zimura may have the potential to prevent progression to geographic atrophy altogether in patients with drusen. These post-hoc analyses should be considered hypothesis seeking. Nonetheless, if these results are substantiated with prospective, randomized studies, the potentially sight-saving impact of Zimura on millions of high-risk patients could be a massive leap forward in treating this disease. Our intent is to study Zimura in earlier stages of dry AMD.”
“The impressive data presented today are consistent with our stated goal to build a franchise to treat all stages of AMD, with the expansion of Zimura’s footprint and the continued development of our HtrA-1 inhibitor, IC-500,” stated Glenn P. Sblendorio, Chief Executive Officer of Iveric Bio.
Webcast Information
A live webcast of the event will be available today, June 18, 2021 from 10:00am to 12:00pm ET under the “Events & Presentations” in the Investors section of the Iveric Bio website at https://investors.ivericbio.com. A replay of the webcast will be posted on Iveric Bio’s website following the presentation and available for at least 30 days.
About Zimura
Zimura (avacincaptad pegol) is an investigational drug product and has not been approved for use anywhere globally. Zimura is designed to target and inhibit the cleavage of complement protein C5 and the formation of its downstream fragments, C5a and C5b. By inhibiting the formation of these fragments, Zimura is believed to decrease or slow the chronic inflammation and cell death associated with the retinal aging process by decreasing the formation of membrane attack complex (MAC) and inflammasome activity, thereby potentially avoiding or slowing the degeneration of retinal pigment epithelial cells. This potential mechanism is the rationale for Zimura as a potential therapy for geographic atrophy secondary to age-related macular degeneration.
About Iveric Bio
Iveric Bio is a science-driven biopharmaceutical company focused on the discovery and development of novel treatment options for retinal diseases with significant unmet medical needs. The Company is currently developing both therapeutic product candidates for age-related retinal diseases and gene therapy product candidates for orphan inherited retinal diseases. For more information on the Company, please visit www.ivericbio.com.
Forward-looking Statements
Any statements in this press release about the Company’s future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company’s strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “future”, “may”, “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. In this press release, the Company’s forward looking statements include statements about the timing, progress and results of clinical trials, including expectations regarding patient enrollment and retention in GATHER2 and the availability of topline data from that trial, the Company’s development and regulatory strategy for Zimura, including its potential development in other forms or stages of dry age-related macular degeneration, , and the potential utility of Zimura and its other research and development programs. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to the progression and duration of the COVID-19 pandemic and responsive measures thereto and related effects on the Company’s research and development programs, operations and financial position, the initiation and the progress of research and development programs and clinical trials, availability of data from these programs, expectations for regulatory matters, reliance on clinical trial sites, contract research organizations and other third parties, establishment of manufacturing capabilities, developments from the Company’s competitors and the marketplace for its products, need for additional financing and negotiation and consummation of business development transactions and other factors discussed in the “Risk Factors” section contained in the quarterly and annual reports that the Company files with the Securities and Exchange Commission. Any forward-looking statements represent the Company’s views only as of the date of this press release. The Company anticipates that subsequent events and developments may cause its views to change. While the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law.
ISEE-G
View source version on businesswire.com: https://www.businesswire.com/news/home/20210618005081/en/
Contacts
Investor:
Iveric Bio
Kathy Galante, 212-845-8231
Senior Vice President, Investor Relations
kathy.galante@ivericbio.com
or
Media:
SmithSolve
Alex Van Rees, 973-442-1555 ext. 111
alex.vanrees@smithsolve.com






