ExoStat Medical, Inc. a medical device company engaged in the research and development of the novel MicroTREND System which non-invasively monitors microcirculatory tissue perfusion in real-time at the bedside announced today the successful completion of the world’s first IRB-approved, ICU observational septic shock study to assess the efficacy and clinical utility of oral mucosal partial pressure CO2 (PomCO2) as a parameter for determining the status of microcirculatory tissue perfusion. The findings of this study concluded that data produced by the MicroTREND could, as an adjunct tool, enhance the value of data currently gathered by macrohemodynamic tools currently considered standard protocol.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250624508102/en/
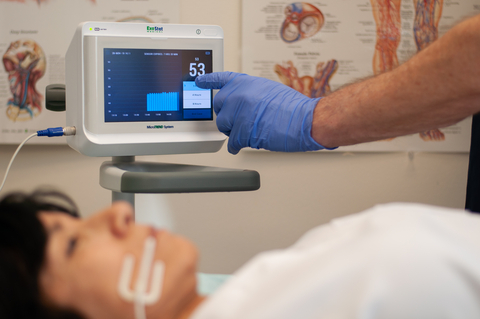
ExoStat's MicroTREND system
Conducted at the Second Affiliated Hospital of Anhui Medical University (Hefei, China) the study enrolled 23 septic shock patients and assessed the role of bedside oral mucosa tissue monitoring PomCO2 as an indicator of early septic shock dysfunction. The study completed a comparative analysis of current standards of care including serum lactate, now considered the gold standard for tissue perfusion evaluation. Principal Investigators of the study are currently preparing the results for near-future publication.
“This first-ever study marks a major milestone for critical care, for ExoStat, and for me as it has been a 40-year project that started with the Weil Institute in the 1980’s,” said Wanchun Tang, MD, MCCM, FAHA, FNAI, and Senior Technology and Medical Advisor for ExoStat. “For the first time ever, physicians were able to view, in real time, the very early and ongoing signs of microcirculatory dysfunction due to tissue hypoperfusion. Every moment matters in septic shock and delays can be devastating to the patient. This device can produce actuals that can provide the physician with more reaction time. It is now well-understood that the current macrohemodynamic tools do not fully capture real-time metrics of the patient under care. This study finally brought what was hypothesis into reality. It was exciting to observe.” Dr. Tang later admitted that it was difficult to see the first measures on the monitor due to tears of joy.
The MicroTREND is a patented, FDA-cleared non-invasive monitoring system that detects PomCO2 using a microsensor that sits atop the inner cheek buccal tissue. There are no needles, no blood, no catheters, and no “lag in time” for lab work required. The importance of time cannot be overstated in treating all shock states, especially sepsis. All shock cases begin with tissue hypoperfusion. It is a common denominator.
“This study achieved the ultimate objective of ExoStat’s mission which was to provide microcirculatory perfusion awareness to physicians who treat the sickest of the sick”, stated Jim Hays, Chief Executive Officer of ExoStat. “We are excited to embark now on our second phase of pre-market clinical testing being planned with iconic research institutions where we will focus on achieving a better understanding of the effect of current standards of care on microcirculation. These are exciting times indeed.”
About ExoStat Medical
ExoStat Medical Inc. is a privately-held medical device company located in Prior Lake, MN. The research and development quest to create the MicroTREND began in 2008 as a vision of Max Harry Weil, MD, who is often called “the father of critical care medicine”. The technology platform developed by ExoStat provides data to enable physicians to assess and to treat microcirculatory hypoperfusion, a life-threatening medical emergency. Visit www.exostatmedical.com for more information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250624508102/en/
"This first-ever study marks a major milestone for critical care and for ExoStat,” said Wanchun Tang, MD, MCCM, FAHA, FNAI.
Contacts
For more information, please contact:
Jim Hays
Chief Executive Officer
jhays@exostatmedical.com






