Clearance extends Pearl’s FDA-validated radiologic AI platform to enable real-time detection of key dental pathologies across the world’s most widely used type of extraoral radiograph.
Pearl, the global leader in AI solutions for dentistry, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for AI-powered detection of dental pathologies on panoramic radiographs. This latest clearance expands Pearl’s clinically validated radiologic AI technology to the most widely captured extraoral imaging modality in dentistry, and is now available to Second Opinion® users in the U.S. and internationally. This clearance marks another major advancement in the company’s mission to elevate diagnostic accuracy, consistency, and efficiency across the full spectrum of dental care.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20251218983939/en/
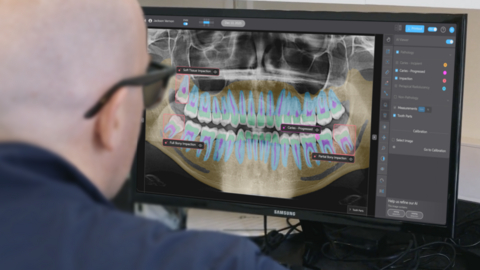
A dentist reviews a panoramic radiograph with support from Pearl’s Second Opinion® AI, now FDA-cleared for automated detection of dental pathologies on panoramic x-rays.
Pearl’s Second Opinion real-time chairside pathology detection platform is now cleared to identify and highlight suspected caries, periapical radiolucencies, and impacted third molars on panoramic radiographs. Panoramic radiographs present a broad anatomical view that can reveal a wide range of dental and maxillofacial conditions—but their complexity and anatomical distortion can make consistent and accurate pathology detection difficult. This latest FDA clearance validates the performance of Pearl’s radiologic AI applied to one of dentistry’s most diagnostically challenging imaging formats.
“Panoramic x-rays are increasingly popular because they capture the full mouth with lower radiation than a traditional full-mouth x-ray series, but they remain one of the hardest types of dental x-ray to read reliably,” said Ophir Tanz, founder and CEO of Pearl. “AI brings greater clarity and certainty to pano interpretation. Patients benefit as well: Diagnoses are easier to understand when findings are clearly highlighted and labeled. Although panoramic x-ray adoption in the U.S. still trails the U.K. and Europe, this FDA clearance affirms the strength of our technology and moves the industry closer to universal AI support across dental radiology.”
The FDA clearance is supported by extensive clinical validation, including a standalone performance study and a fully crossed multi-reader, multi-case (MRMC) study demonstrating that Second Opinion significantly improves detection of caries, periapical radiolucencies, and impacted third molars. Across all study subgroups—including gender, geography, and imaging device type—the AI maintained stable, unbiased performance.
With this clearance, Pearl strengthens its position as the most comprehensive FDA-cleared radiologic AI platform in dentistry, spanning bitewing, periapical, panoramic, and CBCT imaging. For current Second Opinion® users in the U.S. and internationally, panoramic AI support is available both within Pearl’s standalone software and across dozens of imaging and practice management systems where Second Opinion is natively integrated to support seamless adoption within existing clinical workflows.
About Pearl
Pearl is an AI-driven company committed to enhancing patient care in dentistry. Founded in 2019 by a team with decades of experience developing successful, enterprise-grade computer vision solutions, Pearl introduced the first-ever FDA-cleared AI capable of reading and instantly identifying diseases in dental x-rays. With regulatory clearance in 120 countries, Pearl's AI assists dentists in making precise clinical decisions and effectively communicating with patients, thereby transforming the dental care experience worldwide. As dentistry’s global AI leader, Pearl is committed to the ongoing innovation of robust, accessible AI tools that improve patient health outcomes and build greater trust in dental medicine. To request a demo, please visit hellopearl.com/getdemo
View source version on businesswire.com: https://www.businesswire.com/news/home/20251218983939/en/
Pearl’s Second Opinion real-time chairside pathology detection platform is now FDA cleared to identify and highlight suspected caries, periapical radiolucencies, and impacted third molars on panoramic radiographs.
Contacts
Media Contact
Nick Garrison
pr@hellopearl.com
323 438 8330






