The robust, high-quality data and clinical studies supporting the use of Abiomed's (NASDAQ: ABMD) Impella heart pumps in high-risk PCI, cardiogenic shock and right heart failure patients will be showcased at Transcatheter Cardiovascular Therapeutics (TCT) 2021, the annual scientific symposium of the Cardiovascular Research Foundation. The conference will meet virtually and in-person in Orlando, Florida, on November 4 – 6.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211101005297/en/
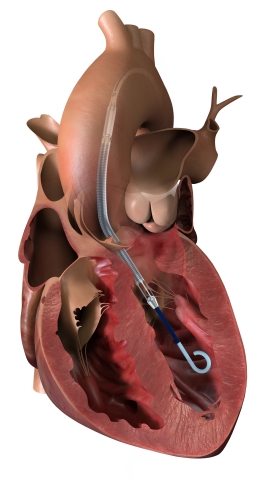
Impella CP with SmartAssist, shown placed in the heart’s left ventricle. (Photo: Business Wire)
Impella heart pumps are the world’s smallest heart pumps. They unload the heart while providing coronary and end organ perfusion. Impella SmartAssist technology enables improved patient outcomes via real-time intelligence. Abiomed augments limited hospital resources by providing 24x7 support on-site, on-call and online.
Throughout TCT, and at a pre-conference workshop on November 3, leading physician-researchers will present data demonstrating:
- Complete revascularization with Impella improves ejection fraction and long-term patient outcomes.
- Cardiogenic shock best practices, including early unloading with Impella pre-PCI and timely escalation to Impella RP and Impella 5.5 with SmartAssist, are associated with improved survival and native heart recovery.
During TCT 2021, physicians will detail final results from two large studies of Impella -- the PROTECT III post approval study and the RESTORE EF study. Interim results from both studies were presented at TCT 2020 and demonstrated the benefits of using contemporary practices to achieve complete revascularization with Impella.
The high-quality clinical evidence being generated from the PROTECT series of studies includes the PROTECT II randomized controlled trial (RCT) and the ongoing PROTECT IV RCT of Impella use in high-risk PCI patients. Based on PROTECT II RCT data, and data from additional robust prospective clinical studies, the United States Food and Drug Administration (FDA) granted Impella premarket approvals (PMA) as safe and effective for high-risk PCI, cardiogenic shock and right heart failure.
TCT presenters will also review the real-world evidence (RWE) and best practices gathered since Impella’s FDA approvals. This evidence will inform the RECOVER IV RCT of Impella use in cardiogenic shock patients. The RWE includes prospective data from the National Cardiogenic Shock Initiative (NCSI) Study, J-PVAD Study and INOVA Study. These datasets demonstrate cardiogenic shock best practices, including early use of Impella, are associated with improved survival rates of 71%, 77% and 82% (respectively), compared to the historical cardiogenic shock survival rate of about 50%.
In-person attendees of TCT 2021 are invited to visit Abiomed at the TCT Industry Hub, which will be open from 8:00 am – 5:30 pm EDT. At the Hub, attendees will be able to participate in hands-on Impella demonstrations, view the newest technologies, talk with members of the Abiomed product development team, and learn the latest information about Abiomed’s ongoing and planned randomized controlled trials.
Virtual attendees of TCT 2021 are invited to participate in three ways:
- Visit www.HeartRecovery.com 24x7 to experience Abiomed’s virtual cath lab and view live and on-demand TCT-related video content.
- Follow the @HeartRecovery and @ProtectedPCI Twitter handles for the latest TCT news and events.
- Tune in for the live TCT recap program, livestreamed on www.HeartRecovery.com at 6:00 pm EDT on Thursday, November 4, and Friday, November 5. Chuck Simonton, MD, and Seth Bilazarian, MD, from the Abiomed medical office will present the top TCT news of the day. After the live broadcast, the program will be available for on-demand viewing.
In-person and virtual participants of TCT are invited to attend a breakfast symposium on Thursday, November 4. The principal investigators of the PROTECT IV RCT, the RECOVER IV RCT and the STEMI Door-to-Unloading RCT will update the physician community on the progress of those trials. The symposium will also review the clinical experience with the new 9 French Impella ECP heart pump. The schedule of presentations and instructions for in-person and virtual viewing are below:
Clinical Science to Landmark Trials of Left Ventricular Unloading in High-Risk PCI and Cardiogenic Shock
Date: Thursday, November 4, 2021
Time: 6:30 – 7:30 am EDT
In-Person Location: Orange County Convention Center, Clinical Science Theater, Level 1, Halls A & B
Virtual Location: Watch via the official TCT 2021 conference livestream
Presenters:
- PROTECT Series Leading to PROTECT IV, Gregg Stone, MD, Mount Sinai Health System, New York City
- Real World Evidence Leading to RECOVER IV, William O’Neill, MD, Henry Ford Hospital, Detroit
- Door to Unload: Implications for STEMI, High-Risk PCI and Cardiogenic Shock, Navin Kapur, MD, Tufts Medical Center, Boston
- Breaking the Small-Bore Barrier: Impella ECP 9 Fr Heart Pump Clinical Experience, Amir Kaki, MD, Ascension St. John Hospital, Detroit
In conjunction with TCT, Abiomed is also hosting a pre-meeting workshop on November 3 for members of CAMP PCI. CAMP PCI (Coronary Artery & Myocardial Protected PCI) is the leading online and in-person physician education platform dedicated to improving patient outcomes and quality of life with supported high-risk PCI by utilizing best practices, techniques and technologies to enable safer, more effective and complete revascularization.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are U.S. FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211101005297/en/
Contacts
Media Contact:
Tom Langford
Director of Communications
+1 (978) 882-8408
tlangford@abiomed.com
Investor Contact:
Todd Trapp
Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com






