- Primary endpoint of QUILT-3.078 is overall survival; median overall survival has not yet been reached, with 19 of 23 enrolled patients alive as of January 22, 2026
- Fourteen patients have evaluable data, with the longest survival from time of disease recurrence reaching 12 months to date and ongoing
- Baseline mean absolute lymphocyte count (ALC) among these 14 patients was 0.9 x 103/uL, confirming severe lymphopenia at enrollment
- ALC increased within one treatment cycle, with mean ALC rising to ≥ 1.4 x 103/uL (p <0.001, N=14)
- Immune competence (as measured by ALC) was maintained, with statistically significant increases from baseline observed at all assessments through 20 weeks (p ≤ 0.026) with ANKTIVA® + CAR-NK
- ANKTIVA + CAR-NK demonstrated a manageable safety profile following a total of 219 doses administered to date, with three treatment-related serious adverse events reported among 41 GBM patients (2L and 3L+) enrolled across QUILT-3.078 (N=23) and single-patient INDs (N=18)
- Randomized controlled trials in both first line (1L) and second line (2L+) glioblastoma are in development
ImmunityBio, Inc. (NASDAQ: IBRX), a commercial-stage immunotherapy company developing cytokine and cellular immunotherapies designed to restore immune competence, today announced updated Phase 2 clinical results from QUILT 3.078 (NCT06061809), evaluating a chemotherapy-free combination immunotherapy regimen in patients with second-line recurrent or progressive glioblastoma (GBM), as well as patients treated under single-patient INDs (spINDs) across first- to third-line disease.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260123454593/en/
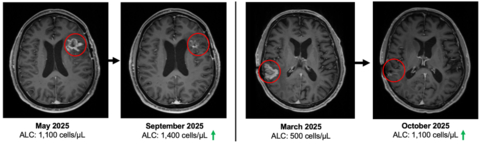
Figure 1
QUILT-3.078 enrolled patients with recurrent GBM at first recurrence. In the spIND population, seven patients were first line and are alive to date. Randomized clinical trials for both first line and second line+ GBM patients are in development.
QUILT-3.078 Phase 2 Results to Date
As of January 22, 2026, the study has enrolled 23 patients with recurrent or progressive GBM who progressed following standard-of-care therapy, including surgery, radiation, and temozolomide-based chemotherapy. 19 of the 23 enrolled patients remain alive, with four deaths reported to date. See figure 1.
Of the 23 enrolled patients, 14 currently have evaluable clinical data and comprise the efficacy population for the current analysis. Among these evaluable patients, a total of 139 doses of combination immunotherapy have been administered. The median follow up is 6 months in the evaluable cohort. All four deaths to date occurred within the evaluable cohort, and median overall survival has not yet been reached. See figure 2.
“Across contemporary studies, median overall survival for patients with recurrent glioblastoma is approximately six to nine months. Outcomes beyond this benchmark remain uncommon, underscoring the substantial unmet medical need in this disease. The patients enrolled in this study represent a particularly challenging population, having progressed after standard therapies and with severe lymphopenia. Observing ongoing treatment durability, survival beyond historical expectations in some patients, and a manageable safety profile without chemotherapy represents a paradigm change in the treatment of patients with glioblastoma,” said Simon Khagi, M.D., MBA, Medical Director of Neuro Oncology at the Hoag Family Cancer Institute and Principal Investigator. Updated clinical findings will be presented by Dr. Simon Khagi at the Stand Up to Cancer Glioblastoma Innovation Scientific Summit on January 31, 2026 in Pasadena, Calif., including the focus on immune agonists as a combination backbone and the ImmunityBio Bioshield regimen.
Immune competence and lymphocyte recovery
At study entry, patients demonstrated immune compromise consistent with prior standard of care, including radiation and alkylating chemotherapy. The baseline mean absolute lymphocyte count (ALC) was approximately 900 cells per µL, consistent with lymphopenia commonly observed after prior therapy in recurrent GBM. See figure 3.
“These Phase 2 data reflect outcomes in a second- and third-line glioblastoma population where immune collapse after standard therapy is common and options are limited. With 19 of 23 enrolled patients alive and median overall survival not yet reached, the survival profile warrants continued follow up,” said Patrick Soon Shiong, M.D., Founder, Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio. “It has been extensively reported that patients with glioblastoma and lymphopenia have a significant decreased survival. Current standards of care, including radiation and chemotherapy (temozolomide), are potent drivers of lymphocyte depletion. The patients that entered this study all suffered profound lymphopenia, reflected by a baseline mean ALC of approximately 900, consistent with prior radiation and chemotherapy exposure. On treatment, we observed recovery and maintenance of lymphocyte counts without chemotherapy. Notably, within this broader clinical and compassionate use experience, we have also observed a near complete response with survival extending beyond twelve months from the time of documented disease progression, an outcome rarely seen in recurrent glioblastoma.”
Safety
The treatment regimen has demonstrated a manageable safety profile. Three participants experienced serious adverse events suspected to be related to the experimental therapy. Importantly, no cytokine release syndrome (CRS) or immune effector cell associated neurotoxicity syndrome (ICANS) was observed.
“Glioblastoma remains one of the most lethal cancers we treat, with five-year survival still below ten percent and little meaningful improvement despite decades of drug development. A central challenge has been the disconnect between therapeutic development and a mechanistic understanding of how these agents behave in the human brain. Lymphopenia has been underrecognized as a significant consequence of our standards of care and the effect of a low ALC level on overall survival,” said Joshua Bernstock, M.D., Ph.D., Clinical Fellow, Harvard Medical School and Neurosurgeon, Brigham and Women’s Hospital. “Approaches that integrate immune-based therapies that maintain immune competence with direct clinical and biological observation are essential if we are to make progress. Efforts that prioritize immune competence, durability of response, and data-driven evaluation represent an important step toward redefining how glioblastoma therapies are developed and assessed. I look forward to developing this new class of lymphocyte-stimulating agent and NK-based cellular therapy in glioblastoma with ImmunityBio.”
About QUILT-3.078 (NCT06061809)
Overall survival is the primary endpoint of QUILT-3.078. The study includes an open-label, single-arm Phase 2 portion evaluating ANKTIVA® (nogapendekin alfa inbakicept) in combination with PD-L1 t-haNK, bevacizumab, and Tumor Treating Fields in patients with recurrent glioblastoma.
The program also includes a randomized Phase 2B component designed to further evaluate durability of benefit. In Phase 2B, patients are randomized 1:1 to receive ANKTIVA, bevacizumab, and Tumor Treating Fields, either with or without PD-L1 t-haNK. Per protocol, study therapy is administered on an every-two-week dosing schedule within protocol-defined treatment periods. A Phase 2B expansion cohort is currently enrolling, with a target enrollment of 20 patients. To date, 8 patients have been enrolled in the Phase 2B cohort. This expansion is intended to further evaluate overall survival, durability of clinical outcomes, and exploratory immune biomarker analyses in this setting.
Single Patient IND (spIND) experience
In parallel with the clinical trial program, 18 patients with recurrent GBM have initiated treatment under a single patient IND, of which seven patients are first line.
About Glioblastoma
Glioblastoma (GBM) is the most common and aggressive primary malignant brain tumor in adults. Despite maximal multimodal therapy consisting of surgical resection, radiation, and temozolomide-based chemotherapy, outcomes remain poor. Median overall survival following initial diagnosis is approximately 14–16 months, and in the recurrent setting, median survival is typically ranges from 6 to 9 months. Five-year survival rates remain below 10 percent, underscoring the limited impact of existing therapeutic approaches.
A defining but underappreciated feature of glioblastoma management is treatment-induced immune suppression, particularly lymphopenia.i, ii Multiple clinical studies have demonstrated that low absolute lymphocyte count (ALC) at baseline or following standard-of-care therapy is independently associated with inferior survival in glioblastomaiii, regardless of tumor burden or extent of resection. Radiationiv and alkylating chemotherapyv have been shown to cause profound and durable depletion of circulating lymphocytesvi, which may impair antitumor immune surveillance and limit the effectiveness of subsequent therapies.
Published analyses have further shown that persistent lymphopenia following chemoradiation is associated with shortened overall survival, while preservation or recovery of lymphocyte populations correlates with improved outcomesvii. These observations have contributed to growing recognition that immune competence is a biologically relevant determinant of survival in glioblastomaviii, and that therapies capable of restoring or sustaining lymphocyte populations may be necessary to enable durable disease control. As a result, glioblastoma is increasingly understood not only as a locally aggressive malignancy, but also as a disease characterized by systemic immune collapse, creating a rationale for treatment strategies that prioritize immune restoration alongside tumor-directed interventions.
About ImmunityBio
ImmunityBio is a vertically integrated commercial stage biotechnology company developing next-generation therapies that bolster the natural immune system to defeat cancers and infectious diseases. The Company’s range of immunotherapy and cell therapy platforms, alone and together, act to drive and sustain an immune response with the goal of creating durable and safe protection against disease. Designated an FDA Breakthrough Therapy, ANKTIVA is the first FDA-approved immunotherapy for non-muscle invasive bladder cancer CIS that activates NK cells, T cells, and memory T cells for a long-duration response. The Company is applying its science and platforms to treating cancers, including the development of potential cancer vaccines, as well as developing immunotherapies and cell therapies that we believe sharply reduce or eliminate the need for standard high-dose chemotherapy. These platforms and their associated product candidates are designed to be more effective, accessible, and easily administered than current standards of care in oncology and infectious diseases. For more information, visit ImmunityBio.com (Founder’s Vision) and connect with us on X (Twitter), Facebook, LinkedIn, and Instagram.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements in this press release include, without limitation, statements regarding: the interpretation, significance, and potential implications of clinical data from the Company’s Phase 2 clinical trial QUILT 3.078 (NCT06061809), evaluating a chemotherapy-free combination immunotherapy regimen in patients with second-line recurrent or progressive glioblastoma (GBM), as well as patients treated under single-patient Investigational New Drug (IND) applications across first- to third-line disease; the potential safety, tolerability, efficacy, and therapeutic profile of the Bioshield regimen in this investigational context; the potential for observed clinical activity to translate into clinical benefit; anticipated future clinical development plans, including the initiation, design, timing, and outcomes of future studies; regulatory interactions and pathways; and the potential role of the Bioshield regimen in the treatment of cancer.
Forward-looking statements are based on the Company's current expectations, assumptions, and beliefs and involve risks, uncertainties, and other factors that could cause actual results to differ materially than those expressed or implied by such statements. These risks and uncertainties include among others: the early and preliminary nature of clinical data; the fact that data from single patient IND use is limited in scope and may not be predictive of results in larger controlled clinical trials; the possibility that interim or topline results may change as additional data become available or as data are further analyzed; variability in patient response, particularly in oncology populations; unexpected safety signals or adverse events; challenges in patient enrollment and retention; the risk that clinical trial results may not support further development, regulatory approval, or commercialization; the timing, outcome, and unpredictability of regulatory review by FDA and other regulatory authorities; manufacturing and supply risks; and competition from existing or future therapies. The investigational product candidate discussed in this press release has not been approved by FDA or any other regulatory authority and its safety and efficacy has not been established.
More details about these and other risks that may impact the Company’s business are described under the heading “Risk Factors” in the Company’s Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) on March 3, 2025, and the Company’s Form 10-Q filed with the SEC on November 5, 2025 and in subsequent filings made by ImmunityBio with the SEC, which are available on the SEC’s website at www.sec.gov. ImmunityBio cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date hereof. ImmunityBio does not undertake any duty to update any forward-looking statement or other information in this press release, except to the extent required by law.
References:
-
Kim WJ, Dho YS, Ock CY, Kim JW, Choi SH, Lee ST, Kim IH, Kim TM, Park CK. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol. 2019 Jun;143(2):321-328. doi: 10.1007/s11060-019-03167-2. Epub 2019 Apr 13. PMID: 30982199.
-
Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016 Apr;127(2):329-35. doi: 10.1007/s11060-015-2037-1. Epub 2016 Jan 4. PMID: 26725885; PMCID: PMC4783226.
-
Byun HK, Kim N, Yoon HI, Kang SG, Kim SH, Cho J, Baek JG, Chang JH, Suh CO. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019 Mar 27;14(1):51. doi: 10.1186/s13014-019-1256-6. PMID: 30917849; PMCID: PMC6436232.
-
Kim N, Lee J, Shin H, Shin J, Nam DH, Lee JI, Seol HJ, Kong DS, Choi JW, Chong K, Lee WJ, Chang JH, Kang SG, Moon JH, Cho J, Lim DH, Yoon HI. Nomogram for radiation-induced lymphopenia in patients receiving intensity-modulated radiotherapy based-chemoradiation therapy for newly diagnosed glioblastoma: A multi-institutional study. Clin Transl Radiat Oncol. 2024 May 22;47:100799. doi: 10.1016/j.ctro.2024.100799. PMID: 38884005; PMCID: PMC11176633.
-
Chemoradiation-Induced Lymphopenia in Glioblastoma Patients and the Influence of Total Radiation Exposure Time. Spina, C.S. et al. International Journal of Radiation Oncology, Biology, Physics, Volume 99, Issue 2, E110 - E111
-
Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012:831090. doi: 10.1155/2012/831090. Epub 2012 Oct 24. PMID: 23133490; PMCID: PMC3486128.
-
Anas Saeed Bamashmos et al. Absolute lymphocyte count in patients with glioblastoma treated with temozolomide chemoradiation. J Clin Oncol 37, e13564-e13564(2019). DOI:10.1200/JCO.2019.37.15_suppl.e13564
- Xi J, Hassan B, Katumba RGN, Khaddour K, Govindan A, Luo J, Huang J, Campian JL. The predictive value of absolute lymphocyte counts on tumor progression and pseudoprogression in patients with glioblastoma. BMC Cancer. 2021 Mar 16;21(1):285. doi: 10.1186/s12885-021-08004-2. PMID: 33726710; PMCID: PMC7968315.
View source version on businesswire.com: https://www.businesswire.com/news/home/20260123454593/en/
Contacts
ImmunityBio Contacts:
Investors
Hemanth Ramaprakash, PhD, MBA
ImmunityBio, Inc.
+1 858-746-9289
Hemanth.Ramaprakash@ImmunityBio.com
Media
Sarah Singleton
ImmunityBio, Inc.
+1 415-290-8045
Sarah.Singleton@ImmunityBio.com






